Peri-infarct spreading depolarization describes a series of propagating electrical potentials that silence brain activity, alter cerebral blood flow, induce cell swelling, and appear during the hyperacute phase of stroke. PID has been shown to accelerate stroke progression, and suppressing PID is known to reduce infarct volume. Although PID is a potential therapeutic target for stroke, the spatiotemporal properties of PIDs remain to be characterized and how reperfusion affects PID signatures is largely unexplored. Optical imaging and EEG/ECoG recording allow identification of PID, but both techniques are depth-limited and cannot provide volumetric tissue information with clinically relevant indices. To date, PID has not been systemically characterized by MRI due to technical constraints. Because PID appears immediately after stroke onset, we have developed a novel photothrombotic model that allows stroke induction inside the MRI system to capture the entire temporal evolution of PID features and stroke progression. Our goal is to quantitatively probe spatiotemporal PID signatures using perfusion and diffusion MRI in an experimental rat model with and without reperfusion. Our model can also induce an ischemic lesion in any predetermined brain area, which opens up new avenues for preclinical stroke research (Kao et al., Neurobiol Dis).
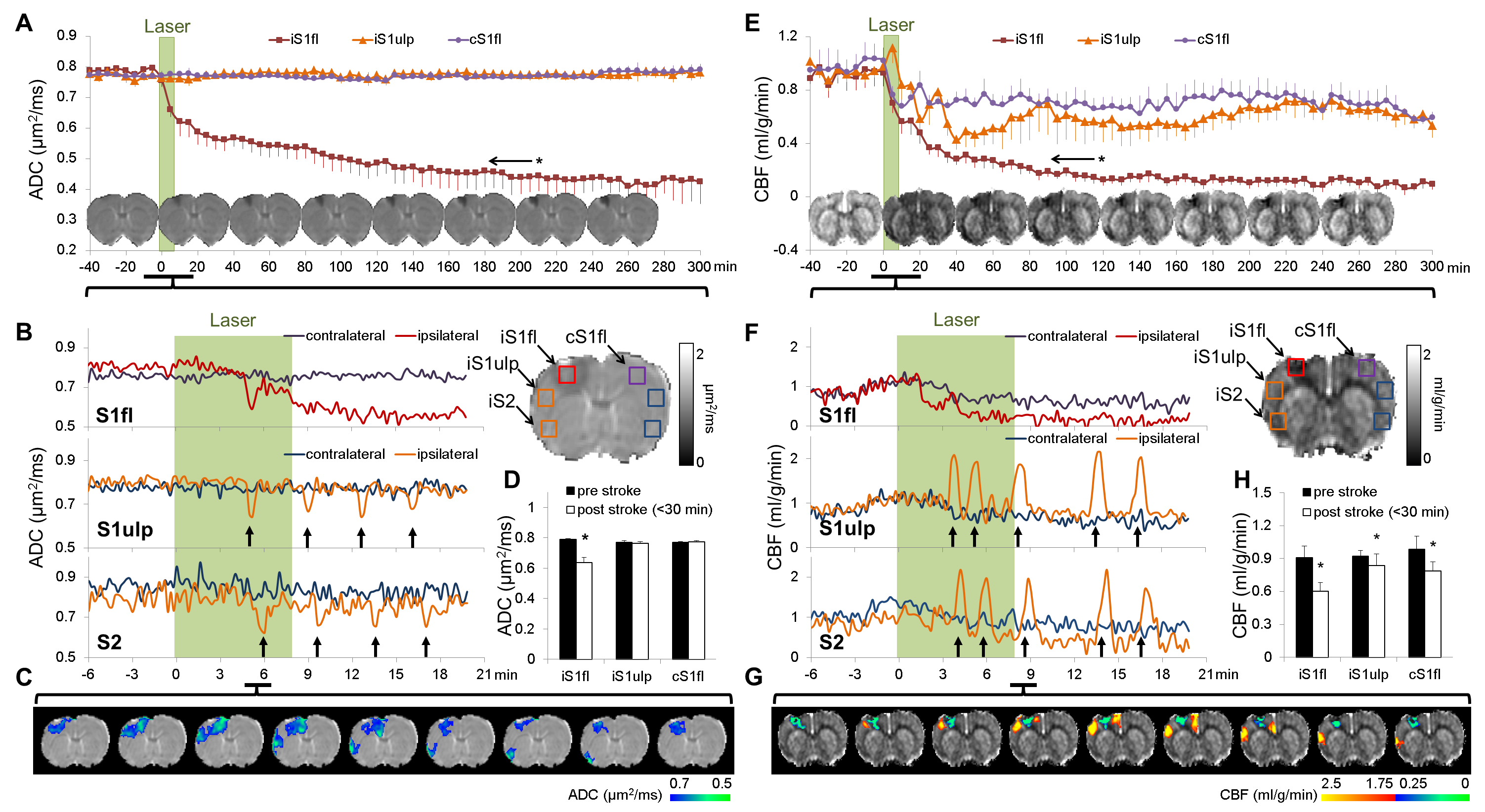
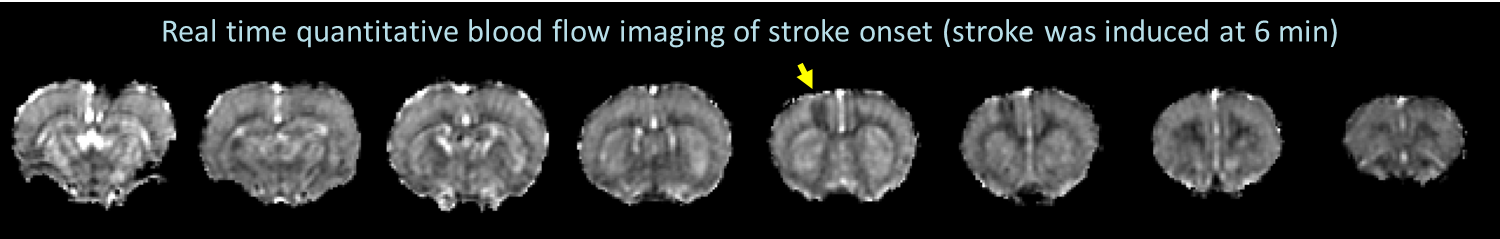
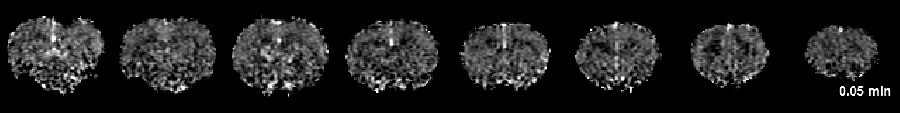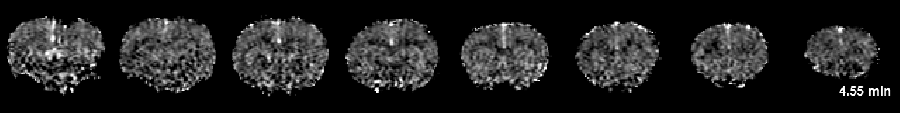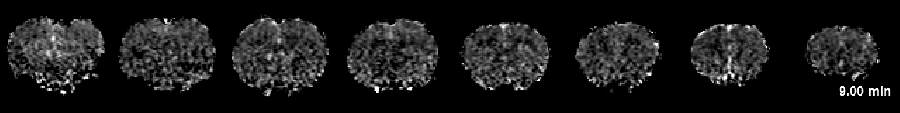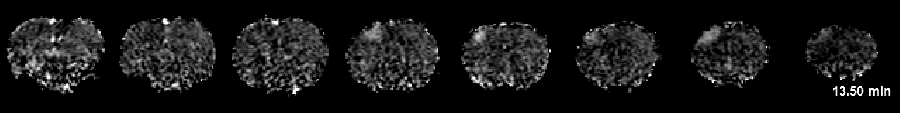
Interestingly, our recent data demonstrated for the first time that intravenous infusion of tissue plasminogen activator (tPA), the only thrombolytic agent approved by the FDA for acute stroke treatment, surprisingly increased the number of PIDs when vascular damage became significant. With these novel tools in hands, we are interested studying the motor behavioral correlates of these imaging signatures, and further address two questions, namely: 1) whether tPA-induced PIDs exacerbate ischemic injury and 2) whether co-administration of PID-suppressing agents during tPA infusion improves outcomes. Our results should shed light on a novel strategy to reduce tPA-induced tissue toxicity. This project, if successful, could ultimately lead to a clinical trial improving the overall efficacy of intravenous tPA treatment in stroke.